Dimethyl ether

One solution to the hydrogen economy of the future might possibly be found in many deodorant spray cans. Dimethyl ether (DME) has long been used as a propellant gas.
Scientists from Forschungszentrum Jülich, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), and the Fraunhofer Institute for Solar Energy Systems ISE have published a paper on the topic of dimethyl ether as a hydrogen storage medium in the renowned journal Energy & Environmental Science. In the paper, they describe the closed DME/CO2 cycle as a “hitherto underestimated” form of hydrogen storage and demonstrate the potential of DME for transporting hydrogen over very long distances. In the article, they explain how the hydrogen transport technology “could significantly impact a future global hydrogen economy”.
Known properties, new application
DME liquefies at low pressure. It is highly flammable and forms carbon dioxide (CO2) and hydrogen (H2), when it reacts with water vapour during the steam reforming process. The liquefaction at low pressure is relevant for applications in deodorant sprays. DME is liquid when under pressure in the spray can. When it is released, it changes to a gaseous state and is therefore a suitable carrier for deodorant fragrances and agents. DME is therefore one of the propellant gases that have replaced chlorofluorocarbons (CFCs), which are harmful to the ozone layer.
“All the properties of DME are known,” says Dr. Michael Alders, who authored the paper and is a member of Forschungszentrum Jülich’s Institute for a Sustainable Hydrogen Economy (INW). Alongside INW, Forschungszentrum Jülich’s Helmholtz Institute Erlangen-Nürnberg for Renewable Energy (HI ERN) was also involved in the paper.
Utilize the advantages of DME
Significantly more usable hydrogen is released per mass of transported DME than is the case with ammonia or methanol. In contrast to ammonia and methanol, DME is also nontoxic and therefore easier to handle. “You can compare the handling of DME with a gas such as butane, which can be stored in a camping gas canister,” explains Michael Alders.
With DME, the temperature required to release the hydrogen (250 °C – 400 °C) is comparable to methanol (250 °C – 300 °C) and lower than ammonia (400 °C – 600 °C). Its volumetric energy density (6.0 kWh/L) is also higher than that of methanol (4.9 kWh/L) and ammonia (4.0 kWh/L). In terms of weight, DME also contains the most energy per kilogram (8.7 kWh/kg) compared to methanol (6.2 kWh/kg) and ammonia (5.9 kWh/kg).
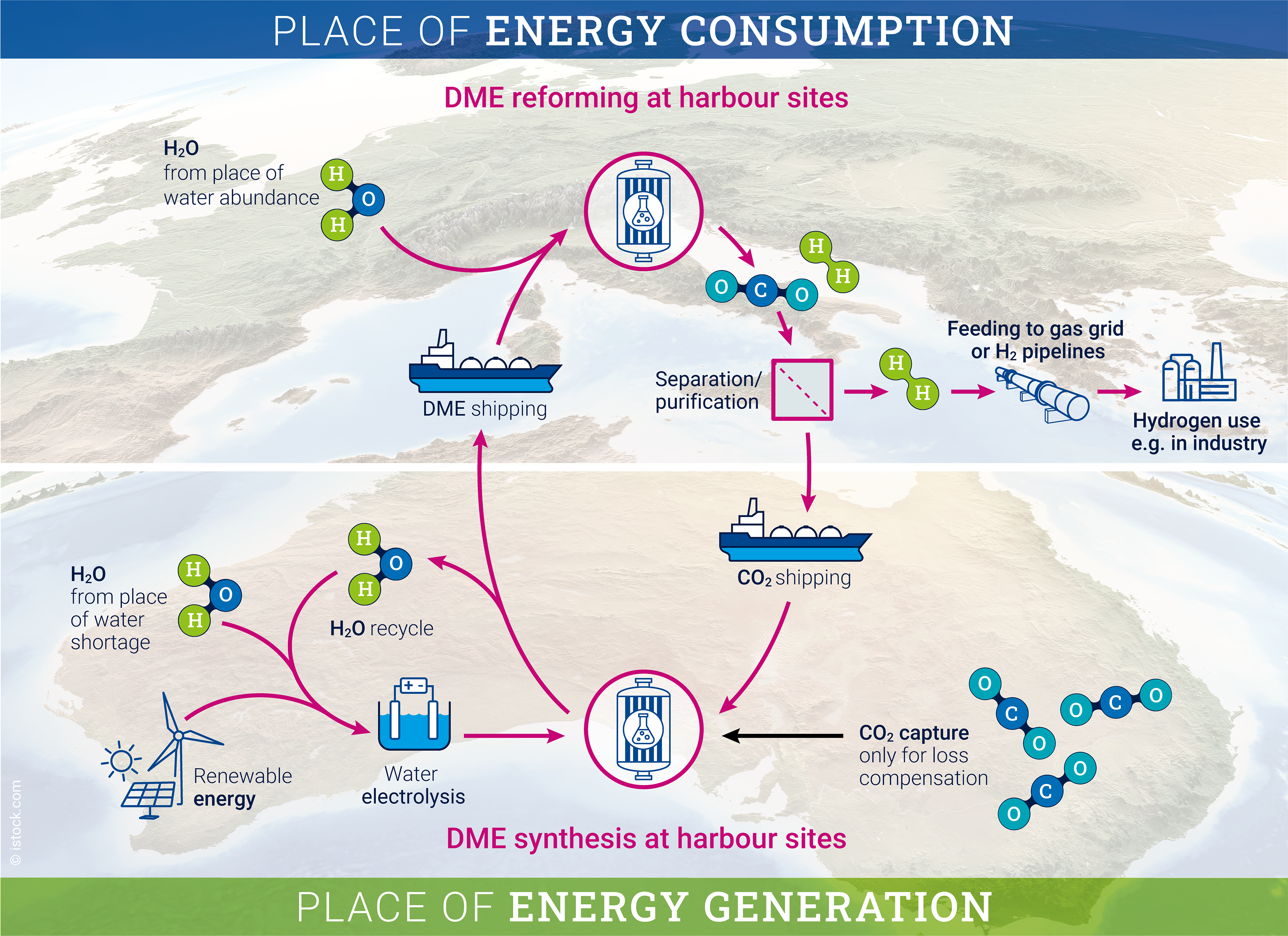
“Although the essential steps of DME-based hydrogen storage are known, they have not yet been combined to form a hydrogen storage technology,” says INW founding director Prof. Dr. Peter Wasserscheid, one of the authors of the paper. “This is something we will work on at INW together with our partners. There is a great level of interest in the DME–CO2 hydrogen storage system in the industry.”
Deposit bottle principle
The authors come to the conclusion that DME is well suited to transporting hydrogen over long distances via sea, for example from South America or Australia – where there is a lot of potential for the production of green hydrogen – to Europe. According to Sebastian Thill (INW), one of the authors of the paper, it would then be conceivable to release the hydrogen at the North Sea ports by means of steam reforming. The second decomposition product after the reaction, CO2, can subsequently be transported back to the hydrogen production sites with the same vessel – similar to the principle of the recyclable deposit bottle – where it can be recombined with hydrogen. “We’re talking about an emission-free cycle in which the CO2 can be used multiple times for hydrogen transport, and is not released into the atmosphere,” says Thill.
INW forms the core of the structural change project HC-H2
Forschungszentrum Jülich’s Institute for a Sustainable Hydrogen Economy (INW) is demonstrating how important hydrogen is as a carbon-neutral energy carrier that is suitable for everyday use to help ensure that the world stops burning fossil fuels. INW forms the core of the Helmholtz Cluster for a Sustainable and Infrastructure-Compatible Hydrogen Economy (HC-H2). Together with its project partners, the cluster is implementing environmentally friendly and economically relevant technologies for the energy economy of the future. These new technologies serve to counteract the phasing out of lignite, providing new and sustainable economic power in the Rhenish mining area.